Small cell lung cancer sclc accounts for about 10 to 15 of all lung.
Pembrolizumab as first line therapy for metastatic non small cell lung cancer.
The two main types of lung cancer are non small cell and small cell.
Among patients with a tumor proportion score for programmed death ligand 1 pd l1 of 50 or greater pembrolizumab has replaced cytotoxic chemotherapy as the first line treatment of choice.
Standard first line therapy for metastatic squamous non small cell lung cancer nsclc is platinum based chemotherapy or pembrolizumab for patients with programmed death ligand 1 pd l1.
First line pembrolizumab monotherapy improves overall and progression free survival in patients with untreated metastatic non small cell lung cancer with a programmed death ligand 1 pd l1 tumour proportion score tps of 50 or greater.
A systematic review of recently published studies.
First line therapy for advanced non small cell lung cancer nsclc that lacks targetable mutations is platinum based chemotherapy.
Each year more people die of lung cancer than die of colon and breast cancers combined.
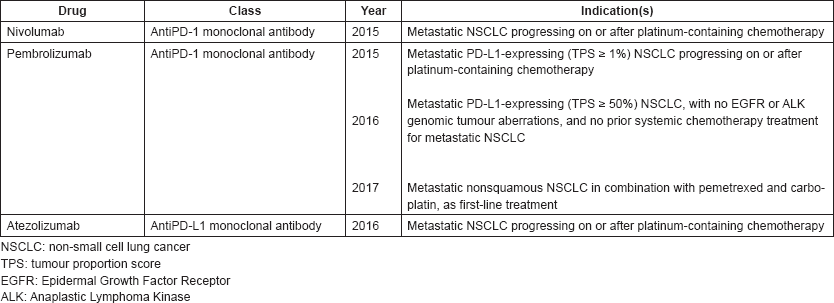
On april 11 2019 the food and drug administration approved pembrolizumab keytruda merck inc for the first line treatment of patients with stage iii non small cell lung cancer nsclc who are.
We investigated overall survival after treatment with pembrolizumab monotherapy in patients with a pd l1 tps of 1 or greater.
Non small cell lung cancer nsclc 80 85 of all lung cancers continues to be one of the major causes of cancer related deaths around the world for the vast majority of patients with advanced or metastatic disease 40 50 of all patients at time of diagnosis platinum based chemotherapy remains the only potential treatment and has led to significantly improved survival outcomes with a.
On august 20 2018 fda granted pembrolizumab keytruda regular approval for the initial or first line treatment of patients with metastatic non small cell lung cancer nsclc whose tumors do not have mutations in the egfr or alk genes.
This review describes trials evaluating the monoclonal antibody pembrolizumab an immunotherapy that blocks the interaction between programmed death 1 and programmed death ligand 1 and 2 pd l1 pd l2 as first line therapy for advanced non small cell lung cancer nsclc.
The change from an accelerated to a regular approval was based on the findings of a large phase 3 trial that included more than 600 patients with.